Switching from fossil fuels to renewable sources of energy is the key to reducing greenhouse gas emissions and achieving the climate protection targets. Particularly renewable electricity has made great progress in recent decades, and the topic of converting renewable electricity into various products (Power-to-X) has been gaining momentum for some years now. In particular, the production of hydrogen via water electrolysis (Power-to-H2) is gaining importance. Yet, how sustainable is hydrogen and its downstream products in the Power-to-X process?
Hydrogen can either be used directly or converted by chemical conversion to other products such as chemicals and fuels. Liquids are very much in demand due to their energy density and the ease with which they can be transported. This process chain is referred to as Power-to-Liquid, or PtL. For Power-to-Liquid processes, hydrogen and one another starting material are required. In most cases, CO2 is used with hydrogen to produce carbon-containing compounds such as methanol. However, the production of ammonia from hydrogen and nitrogen is also moving into the focus. The following figure shows the main processes which we are working on at Fraunhofer ISE.
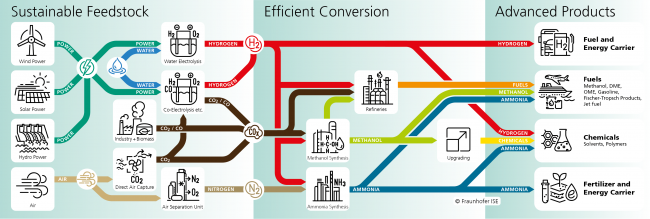
Power-to-H2 and, even more so, Power-to-Liquid processes efficiently convert electricity into products with high energy densities. Thus, renewable electricity can be stored in this form over longer periods of time and can also be transported over long distances. However, these advantages alone do not necessarily mean that Power-to-H2 and Power-to-Liquid processes are sustainable. A precise analysis of the economic, ecological and social aspects is necessary to investigate sustainability.
This text discusses the energy requirements, greenhouse gas emissions, and costs associated with the production of power-to-H2 (hydrogen) and power-to-liquid (PtL) products.
The Energy Required to Produce Hydrogen and PtL Products
In the PtX process, the energy required for hydrogen production is based primarily on the electricity needed for the electrolysis. Additionally, if the hydrogen must be liquefied in order to transport it over very long distances, then this process step also requires a significant amount of energy. The same applies for PtL processes. Because hydrogen is necessary for PtL products, this means that the major factor in energy demand is, in most cases, again the electricity required for electrolysis.
Due to the very high energy density of PtL products, their import, even over long transport distances, requires only very low additional energy. Other energy requirements are due to the heat and electricity supply for the chemical conversion process as well as by the CO2 or the N2 supply. The energy requirements for CO2 and N2 supply are divided into heat and electricity. For Power-to-Liquid processes, it may make sense, especially in future scenarios, to cover the heat requirements with an electricity-powered heat supply in order to achieve the lowest possible greenhouse gas emissions. If the heat demands are met by electric power, then electricity becomes the only relevant input for the production of hydrogen and PtL products.
Therefore, an efficiency value can be determined for the entire power-to-product process. For hydrogen, the efficiency is about 65% without liquefaction and about 55% with liquefaction. For PtL products, the efficiency is between 40% and 55%, depending on the process and the end product, whereby possibilities to increase this efficiency exist, e.g., with heat pumps.
Greenhouse Gas Emissions from Hydrogen and PtL Products
Our work shows that the GHG emissions of both hydrogen and PtL products are mainly influenced by the GHG emissions of the electricity supply [1].
Under the assumption of the complete electrification of the production process as described above, it is possible to calculate what is the maximum amount of greenhouse gas emissions allowed for the electricity generation. This value determines at what point the electricity-powered production of hydrogen or PtL products is equal to the emissions of a fossil-fuel based production.
For hydrogen production, this maximum emissions limit is around 200 to 250 grams CO2 per kWh. For PtL products, this limit ranges from ca.100 to 200 grams of CO2 per kWh [2]. It is only possible to remain below these emissions limits by using renewable electricity, since the fossil-fuel based electricity generation technology with the lowest GHG emissions is a combined cycle power plant, which emits 350 g CO2 per kWh in the best case. Renewable electricity, e.g., from a wind turbine, produces only about 10 to 15 grams CO2 per kWh. Thus, for hydrogen and PtL products it is imperative that the electricity used for production comes almost exclusively from renewable sources. For new Power-to-H2 or Power-to-Liquid processes, moreover, the required amount of renewable electricity should come from power plants constructed additionally for this purpose. As a result, the renewable electricity is not taken away from existing applications and consumers in the power grid, who also need renewable electricity.
Since Power-to-H2 and Power-to-Liquid processes are intended to help reduce greenhouse gas emissions, it is imperative that they have lower greenhouse gas emissions than conventional fossil processes. But beyond that, the processes should also have the potential to be greenhouse gas neutral in the future. Only then will Power-to-H2 and Power-to-Liquid processes be able to help in achieving the long-term climate protection goals. For the processes to be greenhouse gas neutral, the following criteria must be met:
- Greenhouse gas-neutral provision of all electricity and heat requirements
- CO2 must come from renewable sources (e.g., air or biomass)
- Greenhouse gas-neutral production of all plant components
In assessing the future potential of these processes, other environmental impacts such as eutrophication and acidification should be analyzed in addition to greenhouse gas emissions.
Costs of Hydrogen and PtL Products
In contrast to greenhouse gas emissions, the costs of hydrogen and PtL products are not only dominated by the provision of electricity. In addition to the electricity costs, the investment costs also have a decisive share in the total costs.
In the production of hydrogen, electrolysis in particular is responsible for a decisive share of the investment costs. However, if intermediate storage of the gaseous hydrogen or hydrogen liquefaction is necessary, these plants cause non-negligible, additional investment costs.
PtL products generally have higher electricity supply costs and higher investment costs than hydrogen, however, they also have a higher selling price and thus higher added value. This is due to the lower efficiencies and therefore, the associated greater hydrogen demand. In addition, there are investment costs for the CO2 supply and the chemical conversion. One advantage of PtL products is that liquefaction step is usually not required.
In most cases, the electricity supply costs and investment costs for electrolysis account for well over half of the total costs. Therefore, the production of hydrogen and PtL products is particularly promising in areas with low electricity generation costs and high full load hours for renewable energy.
In a scientific article [3], we calculated costs of € 90 per MWh for hydrogen and €128 per MWh for the PtL product methanol for the year 2030, assuming production in North Africa. If transport to Germany is considered, then the costs increase by €25 per MWh for hydrogen and €3 per MWh for methanol. The greatest influence on the costs is therefore not the transport, but rather the electricity price and the investment costs for the electrolysis.
Our work on the sustainability of Power-to-X shows the great potential that hydrogen and PtL products offer for reducing greenhouse gas emissions in the chemical and transport sectors. It also shows potential for further cost reductions.
Further reading
[1] Comparative well-to-wheel life cycle assessment of OME3-5 synfuel production via the power-to-liquid pathway. https://pubs.rsc.org/en/content/articlelanding/2019/se/c9se00658c#fn1
[2] Power-to-What? – Environmental assessment of energy storage systems https://pubs.rsc.org/en/content/articlelanding/2015/ee/c4ee03051f [3] Energy efficiency and economic assessment of imported energy carriers based on renewable electricity https://pubs.rsc.org/en/content/articlelanding/2020/se/d0se00067a











Add comment